▼: ADAM-17 : adhesion : calcium-dependent carbohydrate recognition domains : carbohydrate-presenting molecules : circulatory cells : complement regulatory domains : CRDs : cytokines : ectodomain shedding : EGF : endothelial cells : function : ICAM : inflammatory stimulus : integrins : leukocytes : migration : PECAM : rolling adhesion : saccharide recognition : selectin family : structure : TACE :▼
 Single transmembrane polypeptide selectins occur only in vertebrates, where they undergo calcium dependent binding via their amino-terminal domain to saccharides (sialated glycans). Selectins are one of two families of C-lectins with calcium-dependent carbohydrate recognition domains (CRDs). Lectins are proteins of nonimmune origin that bind to specific carbohydrate structures, distinguishing between different monosaccharides, and specifically binding to oligosaccharides, detecting subtle differences in complex carbohydrate structure.
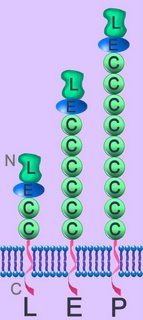
Single transmembrane polypeptide selectins occur only in vertebrates, where they undergo calcium dependent binding via their amino-terminal domain to saccharides (sialated glycans). Selectins are one of two families of C-lectins with calcium-dependent carbohydrate recognition domains (CRDs). Lectins are proteins of nonimmune origin that bind to specific carbohydrate structures, distinguishing between different monosaccharides, and specifically binding to oligosaccharides, detecting subtle differences in complex carbohydrate structure.The selectin family mediates function/migration of circulatory cells: L-leukocytes; E-endothelial cells; P-platelets and endothelial cells. The family possess large, highly glycosylated, extracellular domains, a single spanning transmembrane domain, and a small cytoplasmic tail. They have a single C-type lectin domain (L) at their extracellular amino termini (N). They exhibit homology to complement regulatory proteins, while their N-terminal is homologous to calcium-dependent lectins. Selectins have epidermal growth factor (EGF)-like domain (E) and several complement regulatory domains (C).
L-selectins are expressed on most leukocytes. E-selectins are inducible (transcriptional activation) on vascular endothelium following stimulation by cytokines. P-selectins are found on activated platelets, and their expression is also induced on activated vascular endothelium. P-selectins are stored as preformed transmembrane proteins in cytoplasmic granules, and they are translocated to the cell surface upon stimulation.
Endothelial cells possess ligands for L-selectin, while ligands for E- and P-selectins are found on leukocytes. Selectin-mediated cell adhesion employs carbohydrate recognition of calcium-dependent lectin – each selectin has affinity to oligosaccharides with sialyl-Lex or sialyl-Lea determinants. A mucin-like carbohydrate presenting molecule (PSGL-1) acts as a ligand for selectins. GlyCAM-1, CD34, Sgp200 and MAdCAM-1 act as carbohydrate-presenting molecules for L-selectin. Like most adhesion molecules, the function of L-selectin is regulated by a variety of mechanisms including gene transcription, post-translational modifications, association with the actin cytoskeleton, and topographic distribution.
 Inflammatory stimuli trigger cytokines, which activate endothelial expression of selectins. Rapid binding between selectins and their ligands transiently tether leukocytes at post capillary venules or at high endothlial venules in the lymphoid tissue. The transient nature of selectin-ligand binding allows leukocytes to dissociate and roll along vascular endothelial cells. Inflammatory stimuli trigger the leukocyte adhesion cascade, inducing the expression of integrins at the cell surface.
Inflammatory stimuli trigger cytokines, which activate endothelial expression of selectins. Rapid binding between selectins and their ligands transiently tether leukocytes at post capillary venules or at high endothlial venules in the lymphoid tissue. The transient nature of selectin-ligand binding allows leukocytes to dissociate and roll along vascular endothelial cells. Inflammatory stimuli trigger the leukocyte adhesion cascade, inducing the expression of integrins at the cell surface.Firm adhesion of leukocytes to endothelial cells employs protein-protein interaction between leukocyte integrins and adhesion molecules of the immunoglobulin superfamily (ICAM-1 and VCAM-1). (Above left - click to enlarge) Resting neutrophil (1) within the vascular lumen (vl) possesses surface selectin counter-receptors (s) and inactive integrins (i). Stimulation of an endothelial cell (ec) by an immune stimulus (is) induces the endothelial cell to express selectins, which initially induce rolling adhesion of the neutrophil (2). After activation of neutrophilic integrin expression, the neutrophil adheres firmly (3) to ICAM (I) receptors. Activated neutrophils undergo transendothelial migration (4) into the subendothelial matrix (sem) after adhesion to PECAM-1 (P) at intercellular junctions.
Selectins are rapidly downregulated by proteolytic cleavage. L-selectin is cleaved near the cell surface by ADAM-17 (TACE) and at least one other "sheddase". This process of "ectodomain shedding" causes the release of most of the extracellular portion of L-selectin from the cell surface while retaining the cytoplasmic, transmembrane, and eleven amino acids of the extracellular domain on the cell. E-selectin mediates initial PMN adhesion to endothelial cells, and L-selectin, constitutive trafficking of lymphocytes through secondary lymphoid organs. [L]
Џ beautiful Flash 8 animation - Inner Life of the Cell, which shows rolling adhesion, firm adhesion, and extravasation; Interpretation: Inner Life of the Cell Џ
▲: ADAM-17 : adhesion : calcium-dependent carbohydrate recognition domains : carbohydrate-presenting molecules : circulatory cells : complement regulatory domains : CRDs : cytokines : ectodomain shedding : EGF : endothelial cells : function : ICAM : inflammatory stimulus : integrins : leukocytes : migration : PECAM : rolling adhesion : saccharide recognition : selectin family : structure : TACE :▲
~ cadherins ~ calcium ion סּ cellular adhesion ~ cytokines ~ focal adhesion kinases ~ immunoglobulins סּ Inner Life of the Cell ~ integrins ~ Rho GTPases ~ second messengers ~ selectins סּ signal transduction סּ two-component systems Cell Adhesion Molecules Cell signaling Immune Cytokines Second Messengers Regulatory Proteins Sequences
▲ Top ▲
No comments:
Post a Comment