▼ adhesion molecules : antibodies : antigen binding site : C regions : CD molecules : CDR : chains : complementarity determining regions : co-receptors : costimulation : domains : evolution : Fab : Fc : hinge : ICAMs : immune receptors : immunoglobulin supergene family : integrin binding : isotypes : membrane-bound Igs : signaling : structure : surface markers : V regions : variability generation : VCAM : VDJ recombination ▼
Members of the immunoglobulin supergene family possess two light and two heavy chains linked by both covalent disulphide bridges and by non-covalent forces. They contain conserved amino acid domains that share secondary and tertiary structure. The immunoglobulin supergene family includes:
● antibodies / B cell-receptors (BCRs)
● T cell-receptors (TCRs)
● adhesion molecules ICAM-1, -2, -3, and VCAM
● co-receptors CD4 and CD8
● the costimulatory pairs CD28 and CTLA4 (B7.1 and B7.2)
● all or parts of many other proteins.
The immunoglobulin superfamily is evolutionarily ancient, is widely expressed, and is constitutive or long-term up-regulated. Immunoglobulin antibodies are released by activated B cells of the immune system, on which they also act as surface marker proteins. Adherence of immunoglobilins to foreign substances or to cellular invaders may be sufficient to disarm the invader, or the antibodies attached to foreign substances function as attack signals to macrophages and cytotoxic T cells. Adhesion molecules of the immunoglobulin supergene family signal by activating specific kinases through phosphorylation, resulting in activation of transcription factors, increased cytokine production, increased cell membrane protein expression, production of reactive oxygen species, and cell proliferation.

Immunoglobulins (right - click to enlarge) comprise two heavy chain (h) and two light chain (l) protein subunits, each of which folds into domains (4 on heavy, 2 on light). These adhesion sites or domains contain one or more folds of 60 to 100 amino acids. Depending upon the character of the heavy chain, immunoglobulins are divided into five subclasses or isotypes: IgG, IgD, IgE, IgA, IgM.
Membrane-bound Igs have a transmembrane segment and a cytoplasmic C-terminal tail. The 2 β- chains are stabilized into sandwiched β sheets that are adherent by virtue of hydrophobic interactions between disulphide bonds. Igs assume a Y-shaped structure "topped" at the extracellular N-terminals by variable domains (red), with a variable domain at the tip of the heavy chain (1) and the light chain (2), between which lies and antigen binding site (3). The variable regions are coded by pluripotential DNA sequences that possess the potential to generate thousands of polypeptide sequences capable of adhering to millions of different ligands. Binding is homophilic or heterophilic, including binding to different Igs and to integrins. Both light and heavy chains contain constant domains (white, 4).
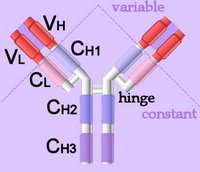 Left - click to enlarge - the heavy chains of IgA, IgD and IgG each have four domains, where those at the N-terminal are variable (VH) and the other three are constant (CH1-3).
Left - click to enlarge - the heavy chains of IgA, IgD and IgG each have four domains, where those at the N-terminal are variable (VH) and the other three are constant (CH1-3).IgE and IgM have one variable and four constant domains (CH1-4) on the heavy chain. The variable domains are termed Fab, while the constant domains are termed Fc. The light chains have two domains, one variable domain (VL) at the N-terminal, and one constant (CL) domain.The antigen binding site lies between VH and VL (shaded lavendar).
Most variability is found in three superficial-loop forming regions in the VH and VL domains, which are the complementarity determining regions or CDRs. CDR3 binds antigens and CDR1-2 bind MHCs. CDR3 shows more variation that do either CDR1 or 2.
The domains have related amino acid sequences that possess a common secondary and tertiary structure. This conserved structure is found frequently in proteins involved in cell-cell interactions and is particularly important in immunology. The constant (Fc) regions have complement fixing and Ig receptor binding activity. The hinge region, in IgG, IgA and IgD, is an important sequence of 10-60 amino acids between CH1 and CH2 that confers flexibility on the molecule.
Immunoglobulins attain their variability by splicing components coded in widely scattered sequences of DNA that are located in two different chromosomes (VDJ recombination). Antigen binding takes place at the heavy chain, which displays enormous variation by virtue of combining 1 of 400 possible variable gene segments with 1 out of 15 diversity segments and 1 out of 4 joining segments. This alternative splicing generates 24,000 possible combinations for the DNA encoding the heavy chain alone. The variable coding segments are assembled together with those for the constant-C segments of the heavy-chain molecule.
· Cadherins · calcium ion · cellular adhesion · cytokines · focal adhesion kinases · Immunoglobulins · Integrins · Rho GTPases · second messengers · Selectins · · signal transduction · two-component systems · Cell Adhesion Molecules Cell signaling Immune Cytokines Second Messengers Regulatory Proteins Sequences
Intercellular adhesion molecule-1 (ICAM-1) expression and cell signaling cascades.
The collective interaction between cells is, in part, mediated by different families of adhesion molecules. Intercellular adhesion molecules (ICAMs) are structurally related members of the immunoglobulin supergene family and are ligands for the beta2 integrins molecules present on leukocytes. Of the five ICAMs identified, ICAM-1 is the most extensively studied. Although ICAM-1 is expressed constitutively at low levels on endothelial cells and on some lymphocytes and monocytes, its expression can be significantly increased in the presence of cytokines (TNFα, IL-1, IFNγ) and reactive oxygen species. Depending upon cell type, ICAM-1 participates in trafficking of inflammatory cells, in cell:cell interactions during antigen presentation, in microbial pathogenesis, and in signal transduction through outside-in signaling events. Again, depending upon cell type examined, ICAM-1 engagement has been documented to activate specific kinases through phosphorylation, resulting in transcription factor activation and increased cytokine production, increased cell membrane protein expression, reactive oxygen species production, and cell proliferation.
Hubbard AK, Rothlein R. Intercellular adhesion molecule-1 (ICAM-1) expression and cell signaling cascades. Free Radic Biol Med. 2000 May 1;28(9):1379-86.
ф activation ~ adhesion molecules ф affinity maturation ~ alternative splicing ф antibodies ф antigen ф antigen presentation ф APCs ф B cells ф BCR ~ cellular adhesion molecules ф complement system ~ cytokines § domains ф evolution of immune and coagulation systems ф humoral immunity ~ immunoglobulins ф isotype switching ♦ kinases ф leukocytes ф leukocyte adhesion cascade ф lymphocytes ф lymphokines ф plasma cells ф receptors ››› respiratory burst ф secondary antibody diversification ф signaling ¤ signaling molecules סּ signal transduction ф somatic hypermutation ф surface receptors ф T cells ф TCR ~ tyrosine kinases ф VDJ recombination :
Immune Cytokines Cell Adhesion Molecules Cell signaling Receptor Tyrosine Kinases (RTKs) Receptor Signal Transduction Second Messengers
▲ Top ▲
No comments:
Post a Comment