Tables Cell Adhesion Molecules Cell signaling Immune Cytokines Second Messengers
▼ cadherins : cadherin superfamily : catenins : cell-ECM : E-cadherin : FAK : families of CAMs : ICAM : immunoglobulin superfamily : importance of cell adhesion : integrins : selectins : signaling ▼
Cell adhesion is important in:
1. maintaining contact within solid tissue
2. embryogenesis (morphogenesis)
3. migration of single cells such as leukocytes within multicellular organisms
4. maintaining contact between neuronal elements
5. virulence of virions and bacteria
There are several families of adhesion proteins, each with specific ligands:
1. integrins with heterophilic attachments to different (hetero) ligands, including selectins with heterophilic attachments to carbohydrate ligands,
2. selectins with heterophilic attachments to carbohydrate ligands
3. Ig superfamily proteins with heterophilic attachments to: a) integrin ligands, and b) Ig superfamily proteins of a different (hetero) type, and homophilic attachments to the Ig superfamily proteins of the same (homo) type
4. cadherins with homophilic attachments to cadherins of the same type.
Cell-extracellular matrix interactions play a central role in tissue architecture and turnover. In particular, integrin-mediated cell adhesion participates in biochemical and physical signals. Cell adhesion molecules of the immunoglobulin and cadherin families, integrin heterodimers, and extracellular matrix glycoproteins, are widely expressed in the nervous system. Adhesion molecules of the immunoglobulin superfamily are involved in cell-cell adhesion, especially important during embryogenesis, wound healing, and the inflammatory response. Cadherins are developmentally regulated, calcium-dependent homophilic cell-cell adhesion proteins. Integrins are heterodimeric molecules that function both as cell-substratum and cell-cell adhesion receptors. LEC-CAMs are cell adhesion molecules with lectin-like domains that mediate white blood cell/endothelial cell adhesion in the leukocyte adhesion cascade. Homing receptors target lymphocytes to specific lymphoid tissue.
Engagement of ICAM-1, a member of the immunoglobulin supergene family, has been documented to activate specific kinases through phosphorylation, resulting in activation of transcription factors, increased cytokine production, increased cell membrane protein expression, production of reactive oxygen species, and cell proliferation.
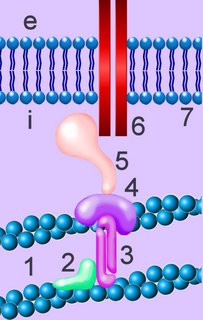 Cadherins are developmentally regulated, calcium-dependent homophilic cell-cell adhesion proteins (right - click to enlarge). The classic cadherins are defined by a conserved intracellular (i) domain which mediates interactions with cytoplasmic proteins termed catenins: α- and β-catenin. β-catenin (5) binds to both the C-terminus of the cadherin intracellular domain (6) and the N-terminus of α-catenin (4). α-catenin binds to a number of proteins involved in actin binding, bundling and polymerisation, as well as binding directly to F-actin (1) of the cytoskeleton, here through α-actin (3) in association with vinculin (2).
Cadherins are developmentally regulated, calcium-dependent homophilic cell-cell adhesion proteins (right - click to enlarge). The classic cadherins are defined by a conserved intracellular (i) domain which mediates interactions with cytoplasmic proteins termed catenins: α- and β-catenin. β-catenin (5) binds to both the C-terminus of the cadherin intracellular domain (6) and the N-terminus of α-catenin (4). α-catenin binds to a number of proteins involved in actin binding, bundling and polymerisation, as well as binding directly to F-actin (1) of the cytoskeleton, here through α-actin (3) in association with vinculin (2).Absence of α- or β-catenin results in defective cell adhesion and failure of cadherin-catenin complexes to associate with the actin cytoskeleton.
Members of the cadherin superfamily are grouped according to the presence of one or more cadherin repeats in their extracellular (e) domains. Arrays of these (approx. 110 residue) domains form the intermolecular surfaces that mediate cadherin-mediated cell-cell interactions. Patel et al., 2003). The extracellular domains of type I and II (chordate) cadherins consist of five cadherin repeats (CRs). Type III (invertebrate and non-mammalian vertebrate) cadherins have variable numbers of CRs and also contain a region termed the primitive classical cadherin domain (PCCD) which, together with variable numbers of EGF-like and laminin G repeats, lies between the CRs and the transmembrane helix.
E-cadherin is the prototypic member of the cadherin transmembrane protein family, and regulates cell-to-cell adhesion by interacting with (homotype) E-cadherin molecules on opposing cell surfaces. E-cadherin's function in cell adhesion is also critically dependent on its ability to interact, through its cytoplasmic domain, with catenin proteins. A diverse collection of defects alter cadherin-catenin function in cancer cells.
FAK:
Some adhesion-mediated events include activation of focal adhesion kinase (FAK) with subsequent alterations in the cytoskeleton and cell morphology, changes in adhesion strength, and changes in cellular responsiveness to mechanical stimuli.[5] Fibronectin and type I collagen binding requires transcription factors (c-Fos and c-Jun) which are important in cell proliferation. A signaling pathway involving the phosphorylation of focal adhesion kinase and mitogen-activated kinases has also shown to be transiently increased in osteoblasts on fibronectin and type I collagen, but not in cells on poly-L-lysine.[6]
Ig superfamily:
The immunoglobulin superfamily is evolutionarily ancient, is widely expressed, and is constitutive or long-term up-regulated. Immunoglobulin antibodies are released by activated B cells of the immune system, on which they also act as marker, surface proteins.
Integrins:
Integrins are heterodimeric proteins so named because they integrate the function of the cell with the outside world.
Selectins:
Single transmembrane polypeptide selectins occur only in vertebrates, where they undergo calcium dependent binding via their amino-terminal domain to saccharides. The selectin family mediates function/migration of circulatory cells and the leukocyte adhesion cascade: L-leukocytes; E-endothelial cells; P-platelets and endothelial cells. The family possess large, highly glycosylated, extracellular domains, a single spanning transmembrane domain, and a small cytoplasmic tail. Selectins are rapidly downregulated by proteolytic cleavage.
Signaling molecules:
Some signaling molecules act as adhesion receptors and cluster in focal adhesions upon ligand binding. (Rho protein). Adhesion receptors promote the assembly of multi-molecular, intracellular complexes providing mechanical stability and generating intracellular signals that influence cell behavior. The functional properties of adhesion receptor complexes include roles in cell and tissue morphogenesis, cell polarity and cell migration. Adhesion receptors play a role in the control of cell proliferation and differentiation, and malfunction is involved in the development of malignancies. Adhesion receptor signaling is linked to endocytosis, gene transcription, cell cycle, apoptosis, epithelial-mesenchymal transition, tumor invasion, and tumor suppressor proteins. A variety of MAP kinase. Focal adhesions are rich in tyrosine phosphorylated proteins, coupling cell adhesion to signal transduction pathways in the cell. Various adhesion receptors, such as integrins, are closely linked to protein kinases and phosphatases. The adaptor protein Grb2 links focal adhesion kinase (FAK) to the Ras pathway when Grb2 is phosphorylated after binding to FAK. The 85 kDa subunit of the PI 3-kinase is also phosphorylated after binding to FAK. Thus, FAK is a key component in the assembly of focal contact structures that influence cytoskeletal organization and signal transduction.
Above/More Detailed Treatment: catenins : FAK : ICAMs : integrins : selectins : LEC-CAMs : adhesion : (adhesion molecules top) : cell signaling : chemotaxis : embryogenesis : ~ ERKs • GPCRs • GPCR families • hormones • Nitric Oxide• neurotransmission • neuronal interconnections ~ PKA, protein kinase A ~ PKC ~ protein kinase A ~ protein kinase C ~ protein tyrosine kinases • phosphotransfer-mediated signaling pathways • Protein Kinase Signaling Networks • receptor tyrosine kinases •• signaling gradients • signal transduction • two-component systems Џ beautiful Flash 8 animation - Inner Life of the Cell, which shows adhesion-signaling, and Interpretation: Inner Life of the Cell Џ animation MAPK signal transduction : animation G-protein :
Tables Cell Adhesion Molecules Cell signaling Immune Cytokines Second Messengers
Vascular endothelial cell adhesion and signaling during leukocyte recruitment.
During inflammation, coordinated expression of cytokine-induced adhesion molecules (CAMs) on postcapillary venular endothelial cells (ECs) regulates leukocyte recruitment. During their recruitment from blood, leukocytes adhere to EC CAMs, activating signaling pathways inside ECs. In a forthcoming paradigm, leukocyte transendothelial migration requires active EC participation, with extracellular adhesive CAM functions mirrored by cytoplasmic domain-dependent intracellular events. These events serve to reorganize the EC actin cytoskeleton. Investigators have visualized this as changes in EC shape, transient opening of EC-EC contacts, and redistribution of CAMs expressed on the luminal EC surface. In this review, we (1) summarize the overlapping extracellular adhesive properties of the 3 EC CAMs most important for leukocyte recruitment during inflammation, namely, E-selectin, vascular cell adhesion molecule, and intercellular adhesion molecule-1; (2) explore the role of these 3 CAMs as signal transducers by identifying the intracellular signals (Ca++, Rho/Rac, and phosphatidylinositol 4,5-bisphosphate) that upon leukocyte engagement, reorganize the EC cytoskeleton and redistribute these apical CAMs, thereby favoring leukocyte recruitment; and (3) describe how CAM-derived signals lead to ezrin-radixin-moesin complex formation and how this complex of plasma membrane-cytoskeleton adapter proteins coordinates CAM-driven intracellular signals with extracellular adhesive CAM functions. This literature review suggests that the cytoplasmic domains of these EC CAMs and their downstream effectors represent new and potentially beneficial intracellular therapeutic targets for treating diseases of the skin.
Kluger MS. Vascular endothelial cell adhesion and signaling during leukocyte recruitment. Adv Dermatol. 2004;20:163-201.
VCAM-1-mediated Rac signaling controls endothelial cell-cell contacts and leukocyte transmigration. [Am J Physiol Cell Physiol. 2003] PMID: 12700137
Structural pathways for macromolecular and cellular transport across the blood-brain barrier during inflammatory conditions. Review. [Histol Histopathol. 2004] PMID: 15024715
Active participation of endothelial cells in inflammation. [J Leukoc Biol. 2005] PMID: 15629883
Endothelial signaling in leukocyte transmigration. [Cell Biochem Biophys. 2003] PMID: 12794270
PECAM-1 isoform-specific activation of MAPK/ERKs and small GTPases: implications in inflammation and angiogenesis. [J Cell Biochem. 2006] PMID: 16440301
See all Related Articles...
▲ Top ▲
No comments:
Post a Comment