 Integrins: Cell to Matrix Binding and Cell to Cell binding.
Integrins: Cell to Matrix Binding and Cell to Cell binding.Integrins are heterodimeric signaling-adhesion proteins so called because they integrate the function of the cell with the extracellular matrix (ECM). Integrins comprise 120-170 kDa (α) and 90-100 kDa (β) subunits, and are found in multicellular organisms. Mammalian integrins include several subfamilies sharing common β subunits that alternatively associate with different α subunits. β subunits have four cysteine-rich repeated sequences, while α subunits bind divalent cations Mg2+ and Ca2+, which are necessary for their adhesive function.
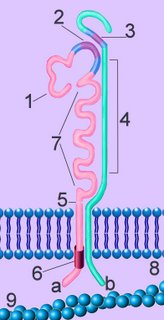
(Right - click to enlarge) The α-subunit (a) comprises a short, membrane penetrating chain that is joined by a sulfide bridge (5) to the long chain, which possesses cation binding sequences (7). The short subunit of the α-chain has a GFFKR site (6) that controls conformational activation. The N-terminus (1) of the α-chain has an I-domain (2). The β-chain (b)includes a corresponding I-like domain (3). The β-chain also crosses the plasma membrane (8), and has four Cys-rich repeated sequences (4). The intracellular domains bind to ligands of the cytoskeleton (9).
Extracellular integrin N-domains bind to ligands such as fibronectin, collagen, laminin, and vitronectin in the extracellular matrix (ECM), usually through recognition of a fibronectin RGD tripeptide [r]. This binding triggers changes in the integrin's cytoplasmic domains, modifying their interaction with cytoskeletal proteins such as talin, paxillin and alpha-actinin, and other proteins that regulate cell adhesion, growth and migration. Signaling functions employ kinases like focal adhesion kinase (FAK) and Src kinase family members to phosphorylate substrates such as p130CAS, thereby recruiting signaling adaptors such as Crk. Signaling is important in cellular growth, division, differentiation, survival, and apoptosis. Along with other CAMs, integrins are important to the modulation of growth cone axon guidance. Cell adhesion molecules of the immunoglobulin (NCAM) and cadherin families, integrin heterodimers, and extracellular matrix glycoproteins, are widely expressed in the nervous system.[n]
Simultaneous to integrin-ECM binding, signals generated within the cell can alter the activation state of some integrins, affecting their affinity for their extracellular ligands. Thus, integrins are able to signal across the membrane in both directions, in-to-out and out-to-in [r] and are important in LFA-1; b, Mac-1; c, p150,95). β2 integrins are expressed exclusively on leukocytes, where they undergo a conformational change accompanied by the phosphorylation of the β subunit upon activation. However, phosphorylation is neither necessary nor sufficient for conformational activation, which is controlled by the GFFKR site immediately adjacent to the transmembrane domain of the α- chain (6).
The β1 integrin subfamily on leukocytes includes Very Late Antigen-4 (VLA-4), which binds to its immunoglobulin ligand VCAM-1, and is chiefly responsible for lymphocyte adhesion to vascular endothelium and leukocyte recruitment to the inflamed area.
(image – conformational changes integrin inactivation) Џ beautiful Flash 8 animation - Inner Life of the Cell, which shows integrins unfolding, and Interpretation: Inner Life of the Cell Џ
· Cadherins · calcium ion · cellular adhesion · cytokines · focal adhesion kinases · Immunoglobulins · Integrins · Rho GTPases · second messengers · Selectins · signal transduction · two-component systems · Cell Adhesion Molecules Cell signaling Immune Cytokines Second Messengers Regulatory Proteins Sequences
▲ Top ▲
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.