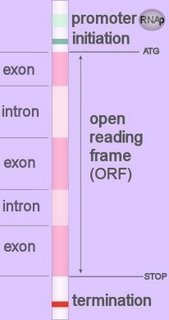
 Introns are segments of DNA that are spliced out of the transcribed pre-mRNA (mnemonic 'in and out').
Introns are segments of DNA that are spliced out of the transcribed pre-mRNA (mnemonic 'in and out').Introns lie between exons in the genome. Introns almost always begin with GT (5' splice donor site) and end with AG (3' splice acceptor site) –this conserved identity of called the 'GT-AG rule'. Sequences adjacent to the initial GT and terminal AG dinucleotides exhibit a considerable degree of conservation (image). Find exons : Find introns : Find gene :
The branch site is involved in formation of a lariat during splicing, and is located within the intron – about 40 nt from the 3' splice site (the terminal AG dinucleotide). The branch site also displays a considerable degree of conservation (image).
Because the exon segments of archival DNA are transcribed and processed into messenger RNAs and translated into proteins, the earlier view of genes held that introns were solely ‘junk DNA’ or DNA 'deserts' because they do not contain ORFs to code for proteins. However, it has recently been recognized that some introns code for micro RNAs and represent a source of epigenetic coding.
Deep intronic mutations causing subtle changes in pseudoexon (located within intronic regions) sequences can activate their inclusion in mature transcripts and cause a genetic disease.
Some segments that were formerly designated introns have been found to contain information for protein, while others code for RNA products and are thus not "junk" DNA. Other introns posses translatable nucleotide sequences that, in the absence of splicing, can generate production of novel peptides (maturases) fused to the peptide encoded by the N-terminal exons. In fungi, these peptide maturases, appear to function in intron removal. Their encoding in introns results in homeostatic regulation of their production. Maturase genes are interspersed within other genes.“
Group I and Group II ribozymes are derived from naturally occurring Group I and Group II introns, respectively. These introns are found in the genes of a variety of lower eukaryotes and prokaryotes. They differ fundamentally from spliceosomal introns since Group I and Group II introns self-splice from the precursor RNA, independent of the spliceosome. The self-splicing intron adopts the catalytic structure that is capable of cleaving RNA splice sites and of ligating the flanking exons together. In addition to self-splicing from RNA precursors, some Group II introns are able to reverse-splice into DNA.
Under certain in vitro conditions, Group II introns can excise themselves from precursor mRNAs and ligate together their flanking exons, without the aid of protein cofactors. The splicing mechanism is essentially identical to splicing of nuclear pre-mRNA introns (pre-mRNA splicing), and this similarity has led to the widespread belief that group II introns were the ancestors of spliceosomal introns, which make up 25% of the human genome.
In addition to self-splicing from RNA precursors, some Group II introns are able to reverse-splice into DNA: they encode ORFs for reverse transcriptase (RT) and are active mobile elements. Such mobile group II introns can insert into defined sites at high efficiencies (called retrohoming), or can invade unrelated sites at low frequencies (retrotransposition).”
Ribozyme-mediated revision of RNA and DNA -- Long et al. 112 (3): 312 -- Journal of Clinical Investigation: modified: "Group I introns are ribozymes that carry out two transesterification reactions in order to excise themselves from a precursor transcript. "
▲ Top ▲
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.